Currently Empty: $0.00
The Oxford Dictionary defines a Fullerene as “a form of carbon having a large spheroidal molecule consisting of a hollow cage of sixty or more atoms, of which buckminsterfullerene was the first known example. Fullerenes are produced chiefly by the action of an arc discharge between carbon electrodes in an inert atmosphere”
Fullerenes are a class of carbon molecules that have a closed or partially closed mesh structure, consisting of carbon atoms connected by single and double bonds. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes 1. The most famous fullerene is buckminsterfullerene (C60), which has a spherical shape and is named after Buckminster Fuller 1. Fullerenes have been the subject of intense research for their chemistry and technological applications, especially in materials science, electronics, and nanotechnology 1
Now while this definition may have you scratching your head (like me) I will try and explain in easier to understand terms in regards to Fullerenes in Shungite.
The first manufactured Fullerenes were made in a laboratory in 1985 by Sir Harold W. Kroto, of the United Kingdom and by Richard E. Smalley and Robert F. Curl, Jr., of the United States. Using a laser in their laboratory, in a helium atmosphere, they vaporised graphite rods and made a cagelike molecule that has 60 carbon atoms.

Structure
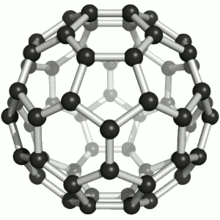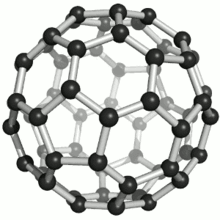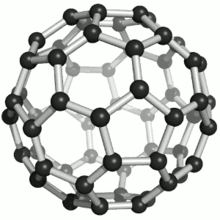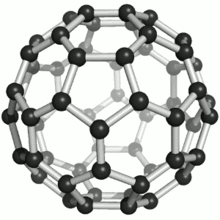
These atoms were joined by single and double bonds with 12 pentagonal and 20 hexagonal faces in the form of a hollow sphere. The C60 Fullerene had been discovered (actually manufactured). At this point in time, no naturally occurring Fullerenes were known to exist.
Fullerenes were named after the American architect Buckminster Fuller for his work on geodesic domes. The Fullerene C60 molecular structure closely resembles his geodesic domes.
A Fullerene C60 molecule is illustrated below (bottom left). It is an extremely strong and stable molecule, naturally occurring only in Shungite.

Shungite with these Fullerenes is only found in one area of the world, The Republic of Karelia in the Russian Federation. This is where Shungite Masters sources all of it products. Shungite deposits have also been found in Eastern Kazakhstan, but the deposit, while in the same class as Shungite, does not contain the Fullerenes.
Analysis
The structure of the carbonaceous matter of black-shale rocks of the “Bolshevik” deposit of the Eastern Kazakhstan region has been investigated by various physico-chemical methods of analysis. It was found to belong to the class of shungites. The authors have established that the Kazakhstan shungite is an amorphous carbon characterized by different morphostructures”
(Yefremova, S.V. & Korolev, Yu.M. & Nauryzbaev, M.K. & Yefremov, S.A.. (2003). The structure of Kazakhstan shungite. Solid Fuel Chemistry. 37. 9-18.)
The C60 Fullerene lends itself to being a good electrical conductor. One of the tests to see if you have a piece of Shungite, and not a piece of coal, is to test its electrical conductivity. Diamonds are bad electrical conductors as are amorphous carbons (like coal). Graphite is a good electrical conductor but has a very soft structure.
Shungite which is rich in Fullerenes has many uses. From EMF protection to being an antioxidant. As the electron in the Fullerene is highly adaptable, it can react easily with radicals, becoming an antiviral agent. As an anti-damage agent for cells to help aging as well various other useful cosmetic things.
We will explore the uses of Shungite in EMF protection in a later blog.


